A .gov website belongs to an official government organization in the United States.
A lock () or https:// means you've safely connected to the .gov website. Share sensitive information only on official, secure websites.
9 March 2023

For decades, atmospheric scientists have been studying and measuring nitrogen oxides – a class of pollutants generated primarily by human activity that are one of the most important contributors to poor air quality.
While research has greatly expanded scientists' understanding of nitrogen oxides, or NOx, the "standard" research-grade instrument used to measure it in the atmosphere hasn't changed much since the 1970s. The size of a major household appliance and weighing several hundred pounds, it requires cryogenic cooling of the electronics to ultra-low temperatures, and is unable to measure NO at levels low enough to answer important scientific questions. Despite these drawbacks, scientists have been hefting them onto research aircraft for nearly four decades to measure NO across the globe.
That has now changed, thanks to a breakthrough design developed by Dr. Andrew Rollins, a research chemist at NOAA's Chemical Sciences Laboratory. Dr. Rollins' design not only measures levels of NO with greater precision at levels 10 to hundreds of times lower than the standard instrument, it's much smaller, lighter, and easier to operate.
"I realized that the measurement uncertainties at lower NO concentrations, even using the existing state-of-the-art instruments, were simply too large to understand all of the sources and chemistry of NO in the atmosphere," said Dr. Rollins about his motivation for developing this new instrument.
Dr. Rollins was awarded a U.S. Patent for his innovative design, which was presented to him in January of this year by Dr. Steven Thur, Assistant Administrator of NOAA's Office of Oceanic and Atmospheric Research. The new instrument, installed aboard NASA's WB-57 research aircraft, is already collecting measurements of NO in the mid-latitude and polar stratosphere during CSL's ongoing SABRE field campaign.
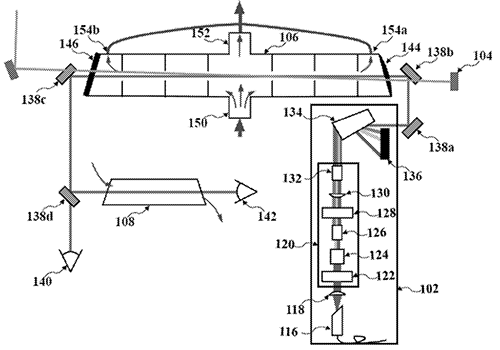
The "standard" research-grade instrument that the scientific community uses to measure NO is essentially the same in form and function as it was in the 1970's when it was first developed for use in the atmosphere. This traditional instrument, which detects NO indirectly using a technique called chemiluminescence. The chemiluminescence technique creates a chemical reaction between NO and ozone (O3) inside the instrument to generate photons (or light) that can be measured by the detector. Since the NO is not being measured directly, other reactions with ozone can lead to detection interferences that can degrade the measurement selectivity.
In contrast, Dr. Rollins' design measures NO directly by using a fiber-optic laser to excite NO molecules at a wavelength of 215 nm, causing them to fluoresce at a wavelength of between 255–267 nm. This technique is known as laser induced fluorescence (LIF). The wavelengths used for the excitation and detection are specific to the NO molecule, which reduces the possibility for interferences, and allows for a much lower detection limit. Indeed, Dr. Rollins' LIF instrument is capable of accurately measuring NO in the atmosphere down to ~0.3–1 parts-per-trillion, 10 to several hundred times lower than the chemiluminescence instruments.
"This instrument is an excellent example of the kind of high-risk, high-reward scientific research at which we excel in CSL," explained CSL Director Dr. David Fahey. "Our innovations in measurement technology lead to ‘disruptive science,' because by pushing the boundaries of what we are able to observe and measure in the atmosphere, we are really advancing science and shifting the paradigms of what we thought we knew."
The technology is unique because it relies on several new approaches to measuring NO, and because the instrument's size, weight, and ability to withstand environmental conditions make it easier to integrate the instrument on a variety of research aircraft for airborne measurements. Dr. Rollins and the NOAA Technology Partnerships Office worked together to write a detailed explanation of how the invention works and submit a patent application to the United States Patent and Trademark Office (USPTO). An invention must be, among other things, useful and new to experts in the field in order to be able to earn a patent under U.S. law.
"Patenting a technology protects the invention as intellectual property and gives NOAA the authority to decide the best approach for driving adoption of the technology," said NOAA Technology Transfer Program Manager, Wayne MacKenzie. The patent protection also provides extra incentive for private companies to invest in licensing and manufacturing inventions. "NOAA patents and then licenses our technologies in order to move new products out of NOAA's labs and into the hands of scientists and other users worldwide, where they can ultimately have broader impact." Holding a patent also allows NOAA scientists to work closely with commercial partners and ensure that technologies are manufactured with high scientific standards in mind.
Despite our extensive understanding of NOx chemistry in polluted environments, a significant knowledge gap exists for regions distant from urban areas where NOx levels are much lower. These so-called "low-NOx" regions are found in forested, marine, polar, and other remote environments, including the Earth's stratosphere (the second layer of the atmosphere above the surface).
"We can quantify lower atmospheric mixing ratios of NO than were possible before, with precision good enough to directly measure a source or sink of NO, even in very clean environments typical of many remote regions of the atmosphere, explained Dr. Rollins. "This matters, for example, in understanding the global lifetime of methane, or understanding how much NO is in the nighttime atmosphere."

Dr. Rollins is currently deploying the NO LIF for CSL's SABRE field campaign – a federally-funded project that has assembled a payload of 17 instruments on-board the NASA WB-57 to precisely measure particles and gases in the stratosphere. The research goal is to better understand the processes associated with proposed climate intervention methods, specifically stratospheric aerosol injection (SAI).
"If we were to increase stratospheric aerosols through SAI," explained Dr. Rollins, "the additional aerosols could lead to increased rates of heterogeneous chemistry involving NOx and halogen compounds, with potential impacts to the ozone layer. That's why it is important to better study this chemistry so we know what the risks could be, and weigh those against any potential benefit of mitigating climate change."
Flights for the SABRE campaign are currently on-going out of Fairbanks, Alaska. A follow-up campaign is planned for next year to study the stratosphere at tropical latitudes.