Chemical Processes & Instrument Development: Instruments
Cavity Ring-Down Spectroscopy (CRDS)
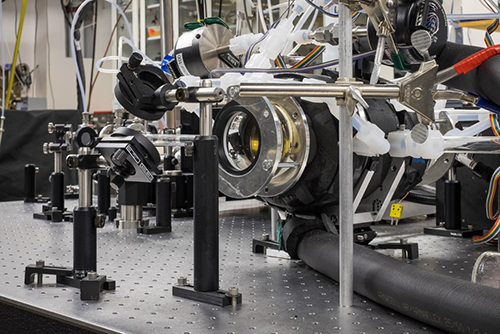
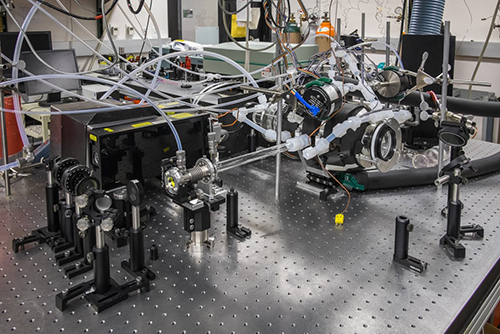
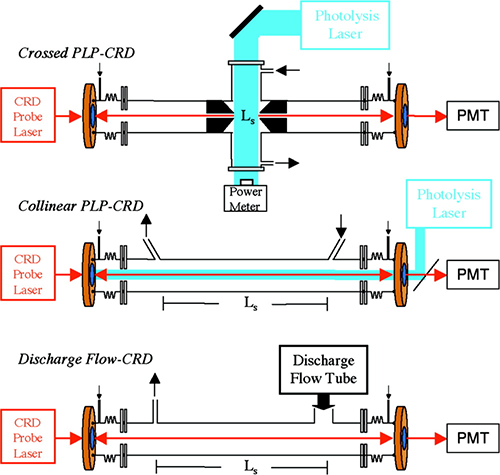
Principle of the Measurement
Cavity ring-down spectroscopy is a spectroscopic technique to measure the extinction (scattering + absorption) of a sample in a high-finesse optical cavity. The measured ring-down time constant, t, is related to the absorption coefficient, α(λ) by:
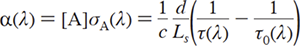
where λ is the CRD probe wavelength, A is the absorbing species, A()is the absorption cross section (cm2 molecule-1) of A at wavelength λ, d is the optical cavity path length (cm), Ls is the path length (cm) of the absorbing sample, c is the speed of light, and τ(λ) and τ0(λ) t0(l) are the ring-down time constants (s) with and without the absorber present.
Technical Specifications
Species Measured: NO3, O3, acetyl radical, halons etc.
Time Response: microseconds
Detection Limit: ppb to ppt (dependent on molecules absorption cross section)
Sensitivity: 0.25 pptv for NO3 at 662 nm
Applications
Temperature and pressure dependent reaction rate coefficient measurements, reaction product yields, absorption spectra, photolysis quantum yields
Key Publications
Papanastasiou, D.K., N. Rontu Carlon, J.A. Neuman, E.L. Fleming, C.H. Jackman, and J.B. Burkholder, Revised UV absorption spectra, ozone depletion potentials, and global warming potentials for the ozone-depleting substances CF2Br2, CF2ClBr, and CF2BrCF2Br, Geophysical Research Letters, doi:10.1002/GRL.50121, 2013.
Gierczak, T., B. Rajakumar, J.E. Flad, and J.B. Burkholder, Kinetic study of the reaction of the acetyl radical, CH3CO, with O3 using cavity ring-down spectroscopy, Chemical Physics Letters, doi:10.1016/j.cplett.2009.11.037, 2010.
Rajakumar, B., T. Gierczak, J.E. Flad, A.R. Ravishankara, J.B. Burkholder, CH3CO radical quantum yields in the 248 nm photolysis of acetone, methyl ethyl ketone, and biacetyl, Journal of Photochemistry and Photobiology A: Chemistry, doi:10.1016/j.jphotochem.2008.06.015, 2008.
Rajakumar, B., J.E. Flad, T. Gierczak, A.R. Ravishankara, and J.B. Burkholder, Visible absorption spectrum of the CH3CO radical, Journal of Physical Chemistry A, doi:10.1021/jp073339h, 2007.