Chemical Processes & Instrument Development: Instruments
Pulsed Laser Photolysis-Laser Induced Fluorescence (PLP-LIF)
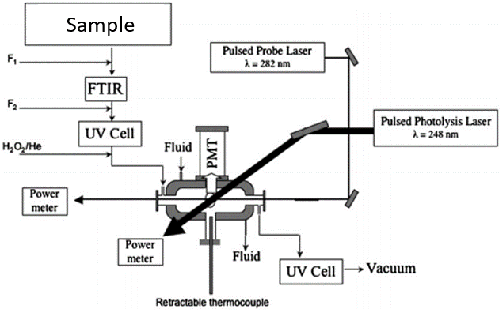
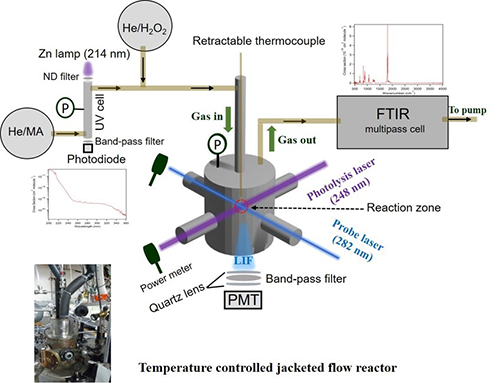
Principle of the Measurement
Pulsed laser photolysis-laser induced fluorescence is the primary method to measure the absolute rate coefficients of OH with various organic compounds. OH radicals are produced by photolysis of a OH-radical precursor, e.g. the photolysis of hydrogen peroxide with a KrF (248nm) excimer laser. A frequency-doubled output of a Nd:YAG pumped dye laser (Rhodamine 590) is subsequently used to excite the resulting OH-radicals (A2∑+ (υ = 1) ← X2Π (υ = 0)). The fluorescence of the excited OH species is measured through a 308nm bandpass filter using a PMT, in the presence and absence of sample. Under pseudo first order conditions where the sample of interest is in large excess, OH temporal profiles are measured by varying the delay between the photolysis and probe lasers. OH temporal profiles measured over a suitable range of substrate concentrations allow the determination of the rate coefficient through a linear-least squares fit of the pseudo first order loss rate versus the concentration of substrate.
Technical Specifications
Species Measured: OH radical, OD radical
Time Response: Delay between photolysis and probe lasers: 10 microseconds to 30 milliseconds
Detection Limit: ~ 5 x 1010 molecule cm-3
Applications
Temperature and pressure dependent reaction rate coefficient measurements, OH photolysis quantum yield
Key Publications
Chattopadhyay, A., V.C. Papadimitriou, P. Marshall, and J.B. Burkholder, Temperature-dependent rate coefficients for the gas-phase OH + furan-2,5-dione (C4H2O3, maleic anhydride) reaction, International Journal of Chemical Kinetics, doi:10.1002/kin.21387, 2020.
Bernard, F., D.K. Papanastasiou, V.C. Papadimitriou, and J.B. Burkholder, Infrared absorption spectra of linear (L2-L5) and cyclic (D3-D6) permethylsiloxanes, Journal of Quantitative Spectroscopy & Radiative Transfer, doi:10.1016/j.jqsrt.2017.08.006, 2017.