Chemical Processes & Instrument Development: Instruments
Quadrupole Chemical Ionization Mass Spectrometer (Q-CIMS)
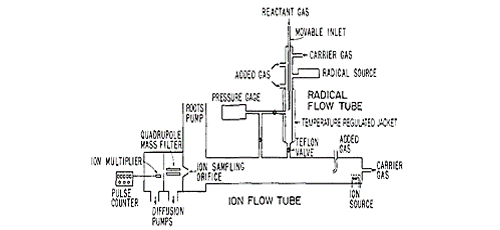
Principle of the Measurement
Chemical ionization relies on the soft ionization of analyte molecules by pre-ionized reactant gas molecules. The collision of these reactant gas fragments and the analyst molecules of interest is tuned to ensure limited fragmentation.
Technical Specifications
Species Measured: small organic molecules of sulfur and nitrogen, halogenated compounds
Detection Limit: ~1 x 109 molecule cm-3, depending on sample
Applications
Absolute temperature and pressure kinetics measurements, gas phase and heterogeneous chemistry: Antarctic ozone hole studies
Key Publications
Longfellow, C.A., A.R. Ravishankara, D.R. Hanson, Reactive and nonreactive uptake on hydrocarbon soot: HNO3, O3, and N2O5, Journal of Geophysical Research, doi:10.1029/2000JD900297, 2000.
Hanson, D. R. Reaction of ClONO2 with H2O and HCl in sulfuric acid and HNO3/H2SO4/H2O mixtures, Journal of Physical Chemistry A, doi:10.1021/jp972767s, 1998.
Hanson, D.R., E.R. Lovejoy, The reaction of ClONO2 with submicrometer sulfuric acid aerosol, Science, doi:10.1126/science.267.5202.1326, 1995.