A .gov website belongs to an official government organization in the United States.
A lock () or https:// means you've safely connected to the .gov website. Share sensitive information only on official, secure websites.
18 February 2020
adapted from the story by NOAA Communications

The discovery of a novel sulfur compound during a historic airborne research mission will likely spur a scientific reassessment of a fundamental marine chemical cycle which drives the formation of oceanic clouds that play a key role in moderating climate, scientists said.
The new chemical, dubbed hydroperoxymethyl thioformate (HPMTF), was discovered by NOAA scientist Patrick Veres while monitoring air samples being analyzed by a new NOAA Chemical Ionization Mass Spectrometer (CIMS) on board the NASA DC-8 airborne science laboratory. The discovery was made on the third of four legs of the NASA-led Atmospheric Tomography Mission (ATom) in September 2017. Observations on the final leg in May 2018 confirmed the finding.
A paper describing the discovery and its impact was published by the Proceedings of the National Academy of Sciences.
"Our new mass spectrometer enabled us to see a sulfur compound no one had identified in the atmosphere before," said Veres, the paper's lead author. "This is a significant development in our understanding of the marine sulfur cycle. It tells us our knowledge is incomplete and we have some work to do to better predict the influence of the marine sulfur cycle on our changing climate."
ATom was a groundbreaking campaign to investigate the impact of human-produced air pollution on greenhouse gases and on chemically reactive gases in the atmosphere.
During ATom, instruments on the NASA DC-8 continuously sampled the atmosphere over the middle of the Atlantic and Pacific Ocean basins, flying a roller-coaster course from the ocean surface to the stratosphere, on four pole-to-pole missions.
One of the ubiquitous chemicals in the marine atmosphere is dimethyl sulfide (DMS). DMS is emitted to the atmosphere by marine phytoplankton. DMS is the most abundant biological sulfur compound, but its concentrations are still low, measured in hundreds of parts per trillion. Reactions involving DMS over the world's ocean basins produces sulfate aerosol, which influences the formation of clouds that in turn affect Earth's climate.
After Veres saw the unexpected readout from the in-flight mass spectrometer, he began investigating immediately after landing. The mass spectrometer had given the researchers a chemical formula for the unidentified molecule. Over the next two years, Veres and colleagues worked to confirm its identity as HPMTF, and developed new laboratory techniques to quantify their observations.
Unbeknown to Veres and his team, researchers from the South China University of Technology had theorized that a new sulfur molecule could potentially be created by reactions involving DMS two years before Veres discovered it in the marine atmosphere. While Veres was writing his paper in 2019, a team of European scientists announced they had created HPMTF from DMS in a laboratory setting.
Still, the confirmation of HPMTF from measurements during the ATom mission has startled scientists. "To me this is a big surprise," said NOAA scientist Patricia Quinn, who leads the atmospheric chemistry research program at the NOAA Pacific Marine Environmental Laboratory and has extensively studied marine aerosols. Quinn was not involved in the study.
"People thought the sulfur budget was well understood," she said. "This throws a kink in the whole works."
With the help of colleagues at the University of Wisconsin and the Institute of Physical Chemistry Rocasolano in Madrid, Spain, the team developed a computer model to explain the results obtained using the ATom data. They estimate that more than 30 percent of oceanic DMS forms HPMTF after it is emitted into the atmosphere, revealing HPMTF to be a major and unrecognized reservoir of marine sulfur that is not currently represented in scientific descriptions of DMS chemistry. It also implies a 60 percent reduction in what scientists thought was a direct line between DMS formation and sulfur dioxide, which is in turn transformed into aerosol particles that influence cloud formation.
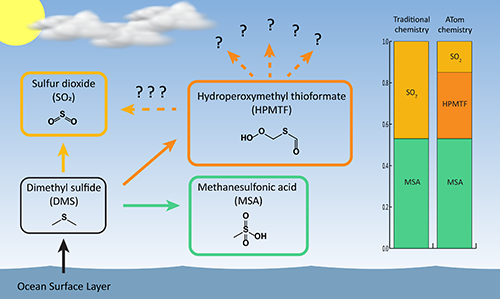
Veres said many questions still remain, and that the degree to which the discovery of HPMTF and future research into its chemistry will alter our understanding of the marine sulfur cycle is unclear. For example, the life cycle of HPMTF in the atmosphere is unknown, limiting scientists' ability to incorporate this chemistry into atmospheric descriptions of sulfur oxidation in the remote marine atmosphere.
"Right now, we're working in the laboratory to better understand the chemical fate of this molecule – the rate at which it forms and the rate at which it reacts to form other molecules in the atmosphere," Veres said.
This knowledge is critical to accurately represent the trajectory of sulfur through the DMS reaction process to refine climate models, which must accurately represent the influence of cloud cover over vast reaches of the ocean.
Fortunately for researchers, Veres said, the ATom campaign has provided an unprecedented dataset of atmospheric observations that will provide a global-scale reference against which future developments in our understanding can be tested for years to come.
"It's pretty interesting that we've been blind to a molecule that has similar concentrations as DMS," Veres said. "That's a big piece of the sulfur pie. Hopefully this will motivate a reexamination of DMS and lead to improvements in our understanding of key linkages between the ocean and clouds, and their combined effects on climate."
The ATom mission was supported through the NASA Earth System Science Pathfinder (ESSP) Earth Venture program. Other members of the research team included scientists from NOAA, CIRES, NASA, and the National Center for Atmospheric Research (NCAR), from the universities of Colorado, Maryland, California-Irvine, Copenhagen and Vienna, and from Penn State University.
Veres, P.R., J.A. Neuman, T.H. Bertram, E. Assaf, G.M. Wolfe, C.J. Williamson, B. Weinzierl, S. Tilmes, C. Thompson, A.B. Thames, J.C. Schroder, A. Saiz-Lopez, A.W. Rollins, J.M. Roberts, D. Price, J. Peischl, B.A. Nault, K.H. Møller, D.O. Miller, S. Meinardi, Q. Li, J.-F. Lamarque, A. Kupc, H.G. Kjaergaard, D. Kinnison, J.L. Jimenez, C.M. Jernigan, R.S. Hornbrook, A. Hills, M. Dollner, D.A. Day, C.A. Cuevas, P. Campuzano-Jost, J. Burkholder, T.P. Bui, W.H. Brune, S.S. Brown, C.A. Brock, I. Bourgeois, D.R. Blake, E.C. Apel, and T.B. Ryerson, Global airborne sampling reveals a previously unobserved dimethyl sulfide oxidation mechanismin the marine atmosphere, Proceedings of the National Academy of Sciences, doi:10.1073/pnas.1919344117, 2020.
Dimethyl sulfide (DMS), emitted from the oceans, is the most abundant biological source of sulfur to the marine atmosphere. Atmospheric DMS is oxidized to condensable products that form secondary aerosols that affect Earth's radiative balance by scattering solar radiation and serving as cloud condensation nuclei. We report the atmospheric discovery of a previously unquantified DMS oxidation product, hydroperoxymethyl thioformate (HPMTF, HOOCH2SCHO), identified through global-scale airborne observations that demonstrate it to be a major reservoir of marine sulfur. Observationally constrained model results show that more than 30% of oceanic DMS emitted to the atmosphere forms HPMTF. Coincident particle measurements suggest a strong link between HPMTF concentration and new particle formation and growth. Analyses of these observations show that HPMTF chemistry must be included in atmospheric models to improve representation of key linkages between the biogeochemistry of the ocean, marine aerosol formation and growth, and their combined effects on climate.