Atmospheric Composition & Chemical Processes: Instruments
Ozone Photometer
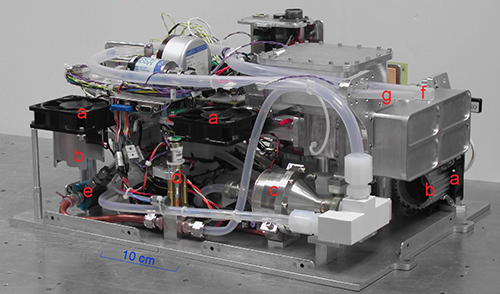
Ozone (O3) is a key atmospheric trace gas that is produced and transported throughout both the troposphere and stratosphere. O3 in the stratosphere is formed from the photolysis of oxygen. Stratospheric O3 (the ozone layer) protects the biosphere by absorbing harmful ultraviolet (UV) radiation from the sun, and this absorption produces the heating that leads to the thermal structure of the stratosphere. Due to the strong gradients of O3 near the tropopause, measurements of O3 are useful in characterizing the chemical nature of air masses and elucidating the dynamics of the upper troposphere and lower stratosphere, including strat-trop exchange and convective redistribution.
- Data rate: 2Hz
- Accuracy: 3%
- Precision with sample flow: 1.1 x 1010 O3 molecules cm-3
- Size: 48cm x 34cm x 22cm
- Weight: 18 kg
- Power (28 VDC): ~200 W (peak); ~50 W (typical)
- Data transmission bandwidth: < 1 kB/s (UDP)
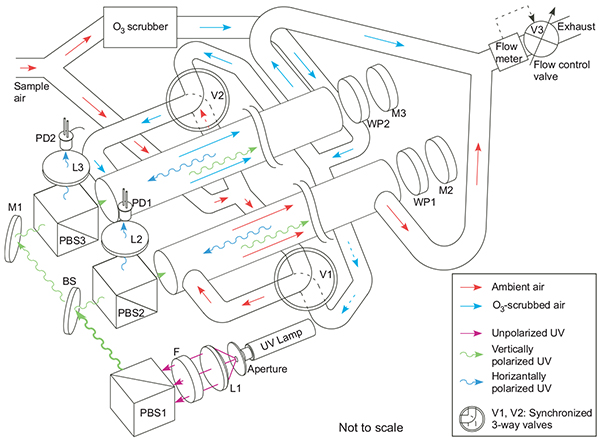
Ambient and O3-scrubbed air flows are directed alternately into two identical absorption cells. 253.7-nm UV light from a mercury lamp is split into two beams that are directed into the absorption cells. Since O3 strongly absorbs photons at 253.7 nm, the UV beam passing through the cell containing ambient ozone is attenuated more that the beam passing through the scrubbed cell. Knowing the O3 absorption cross section (s) and absorption path length (L), the O3 partial pressure (pO3) can be obtained using Beer's law. In our photometer design, a novel optical system was developed that folds the UV beam inside the absorption cells and, thereby, doubling the optical path for a given physical cell length. To achieve this, the unpolarized output of the Hg lamp is collimated by a lens and passed through a 254-nm bandpass filter before entering a polarizing beam splitter (PBS1). The vertically polarized component is then split into two beams using a non-polarizing polka-dot beam splitter (BS) and mirror (M1) combination, with half of the light entering each of the absorption cells unimpeded through another polarizing beam splitter (PBS2 and PBS3, respectively). On the distal end of each cell, the polarized light is reflected and rotated by 90° using precision quarter-wave plate and mirror combinations (WP1, M2 and WP2, M3). After the return pass through the absorption cells, the now horizontally polarized light is reflected into silicon photodiode detectors by PBS2 and PBS3.
- Stratospheric Aerosol processes, Budget and Radiative Effects (SABRE) 2022, NASA WB-57
- Asian Summer Monsoon Chemical & CLimate Impact Project (ACCLIP) 2021, NASA WB-57